What do you think of when someone mentions nitrogen? Maybe it would prompt the thought that it is fundamental to feeding the world’s eight million people. But it would come as no surprise if most of us would not know that for over a century, we have been “fixing” more and more nitrogen from the atmosphere and accumulating it in our soils and waters.
“Fixing” nitrogen means turning the element’s chemically inert gaseous form, which makes up 78% of the atmosphere, into a more reactive compound useful for industry and agriculture.
The mixture of temperature, pressure and catalysts needed in order to make this happen was first produced at industrial scale in Germany in 1913 using the Haber-Bosch process which turned inert nitrogen into ammonia.
Ammonia has many uses; most importantly it can be used to make fertilizers. Without the Haber-Bosch process it is estimated that 30-50% of the world’s harvest would be lost.
And just as the fossil-fuel use has rearranged the planet’s carbon cycle, so the drive to fertilize has upended its nitrogen cycle. About 90% of the nitrogen fixed into fertilizer fails to get into humans. Much of it builds up in the environment rather as carbon dioxide builds up in the atmosphere. As with the carbon dioxide, this causes problems. It kills people, reduces biodiversity and affects the climate.
Some plants – the legumes – build up symbiotic nodules called rhizomes in which the nitrogen-fixing bacteria operate to turn nitrogen into a form the plant can use. In the early 19th-century, crop rotations included legumes as a way of restoring nitrogen. But this was not fully understood or monitored at the time.
By the mid-19th Century, greater crop yields were necessary to sustain growing populations. And so started the importation into Europe of nutrient-rich guano (bird droppings) from pacific islands. When they were depleted, agriculturalists turned to nitrates (another usable form of nitrogen) from Chile. But some estimated that this source would only last another generation. It was only with the arrival of the Haber-Bosch process, that supply appeared limitless.
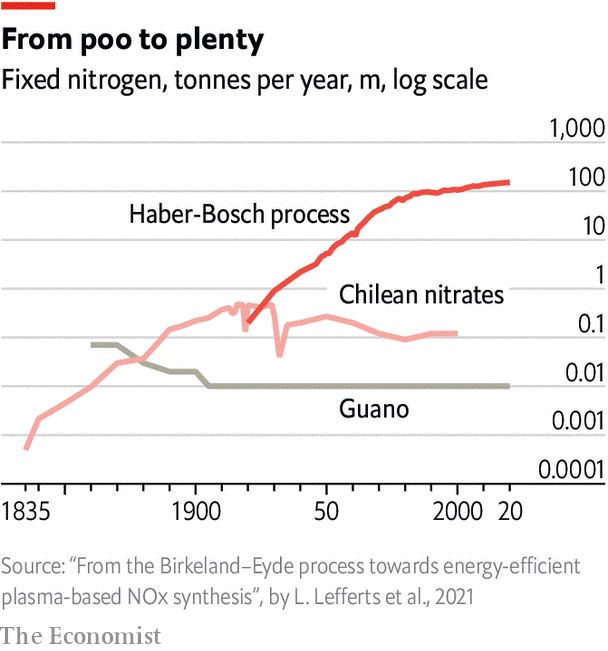
By the mid-19th Century, greater crop yields were necessary to sustain growing populations. And so started the importation into Europe of nutrient-rich guano (bird droppings) from pacific islands. When they were depleted, agriculturalists turned to nitrates (another usable form of nitrogen) from Chile. But some estimated that this source would only last another generation. It was only with the arrival of the Haber-Bosch process, that supply appeared limitless.
With the yield gains from guano, nitrates, and nitrogen fertilizers, and the gains in agricultural productivity from the mechanisation of field work by tractors, combine harvesters and the like, farms became more like factories.
But it was in the second half of the century, when the world’s population was growing exponentially and the risk of famine was high, that nitrogen really came into its agricultural own. The biggest factor in this was that plant breeders—notably Norman Borlaug, working in Mexico for the Rockefeller Foundation—learned how to breed crops particularly adept at growing in highly fertilized soils.
Crops better at using nitrogen drove demand for nitrogen and other fertilizers; abundant nitrogen drove demand for crops better at using it. As new strains became available, yields and nitrogen use climbed; for example yields in India more than tripled between 1960 and 2000. By the end of the century the researchers were finding that roughly 80% of the new varieties’ yield was down to nitrogen fertilizer.
Human industry now fixes about 150 million tonnes of nitrogen every year; more than all the bacteria in all the soils of all the world.
Without human interference, the natural nitrogen cycle balances itself; over time the amount of nitrogen fixed from the atmosphere by bacteria equals the amount of nitrogen stripped out of compounds in the soil and water and returned to the air as nitrogen gas by “denitrifying” bacteria.
But In our eagerness to fertilize the land we have substantially increased nitrogen fixation around the world while doing nothing to increase the rate of denitrification: it currently runs at around 40% of the rate at which nitrogen is added to the environment. As a result, fixed nitrogen is building up in the planet’s soils and waters, just as carbon dioxide is building up in its atmosphere.
Unlike the carbon dioxide in the atmosphere, the nitrogen in the soil does not just accumulate. It builds up in particular places; it acidifies soils and it changes its chemical form. It might return to the atmosphere as ammonia, or as nitrous oxide which is harmful to human health; it might dissolve in run-off as nitrate and travel through streams and rivers to the sea.
The most spectacular examples of this effect are the dead zones now frequently found where rivers that drain large agricultural basins flow out into the sea. Nitrogen and phosphorus from farmlands stimulate exponential growth in some species of algae, which bloom until they have used up all the oxygen in the water. Over the second half of the 20th century such dead zones became ten times more common as nitrogen flows into the sea grew by about half.
According to the Stockholm Resilience Centre, the safe planetary boundary for nitrogen flows had already been significantly breached by 2009.
Another aspect of this superabundance of nitrogen on land is the reduction of biodiversity. A principal reason for this is that some plants are better at using nitrogen than others. If nitrogen levels are increased the plants that are good at using it get a bigger boost than the rest, and outcompete them. Overall biomass may well increase; biodiversity does not.
What to do? You cannot feed eight billion people without providing them with nitrogen, and choices made about how that nitrogen is provided will change life off farms as well as on it—not least because, if you use less nitrogen, you will ordinarily tend to use more land.
One place to start is to recognize that a world which ate fewer animals would have less of a problem, because the conversion of plant protein to animal protein is notoriously inefficient. For example 60% of all arable land today is used for livestock or feed crops. My post on Disrupting the Cow illustrates the far-reaching environmental and health benefits that this shift in diet would bring.
Another more elegant solution is to enlist nitrogen-fixing bacteria to help out. An exciting example comes from American company Pivot Bio which has produced corn seeds coated with highly specific strains of nitrogen-fixing bacteria which provide the plant’s nitrogen requirements. Pivot Bio are not alone. Gingko Bioworks is working on a similar concept utilising microbes that can be coated onto seeds before planting.
Ultimately the solution will likely be a combination of measures including much more efficient use of nitrogen fertilizer. But the speed of implementation will depend on how soon we acknowledge that we actually have a nitrogen problem. It seems way off the radar at the moment.
Only when we build sufficient political will, can we move more quickly toward solutions. As individuals we can make a difference by raising the issue with our elected officials, by reconsidering our diet choices and by supporting farmers who operate on a low nitrogen regime.
As a minimum we need to start talking about nitrogen.
The writer is a co-author of Court of the Grandchildren, a novel set in 2050s America.
ACKNOWLEDGEMENT: This post draws directly and extensively from The Economist essay: The wibbly-wobbly circle of life. The Economist, December, 2022
IMAGE CREDIT: Walter Frehner via Pixabay
